
OR
COVID-19: Clinical Significance of Ct value
Published On: November 12, 2020 07:00 PM NPT By: Sandeep Thapa

Sandeep Thapa
The author is Research Officer at Kathmandu Center for Genomics and Research Laboratory (KCGRL)news@myrepublica.com
COVID-19 was first reported in Wuhan, China in December 2019 and subsequently spread globally, with the declaration of the pandemic in early March 2020. The first confirmed imported case in Nepal was reported in the 2nd week of January 2020. The initial response of Nepal to COVID-19 was comparably slow but the country geared efforts after it was declared a 'global pandemic' by WHO on 11 March 2020.
The government of Nepal steps from 18 March 2020 led to a partial lockdown and a countrywide lockdown was imposed on 24 March 2020. Eventually, this resulted in the rapid and widespread development and implementation of an excess of tests and platforms for the detection of SARS-CoV-2, the virus responsible for this newly emerged disease.
In response to the condition, the government of Nepal implemented Rapid Diagnostic Test (RDT) hoping that its application for testing COVID-19 will assist in screening and end up causing more fatalities. However, public health experts warned that it is a waste of public money and urged the government to focus on ramping up the Polymerase Chain reaction (PCR) test instead. Besides, epidemiologists, public health professionals, and doctors had counseled the government about the ineffectiveness of the RDT.
Subsequently, the ministry allowing private laboratories to conduct polymerase chain reaction tests to diagnose coronavirus patients was one of the prominent steps in the wake of criticism for failing to ramp up PCR tests. Thereafter, Nepal also issued new guidelines for foreign tourists arriving in the country, including carrying a PCR test report conducted not more than 72 hours ago, to minimize the possible spread of the coronavirus infection. Along with the report, the traveler must have booking-documents for the hotel wherein they will stay in quarantine. Until now, nearly eighty (80) which include several hospitals and laboratories, are authorized by the government of Nepal to conduct PCR tests for COVID-19 in Nepal. These all are permitted after assessing the capacity of laboratories in terms of human resources and bio-safety level.
Testing has proven critical to the pandemic response with early detection, subsequent public health, and clinical interventions to both patient and outbreak management. With advances in testing and technology there have been advances in understanding both the disease and its epidemiology. For example, through testing it has become apparent that persons may test positive (i.e., SARS-CoV-2 detected) without symptoms, including for those who are asymptomatic (i.e., never develop symptoms) or presymptomatic (i.e. later develop symptoms). So far, a total of 1.5 million PCR tests have been carried out in Nepal with positive reports of about 200,000 cases.
A term related to the 'gold standard' RT-PCR tests to detect the novel coronavirus has got a lot of attention – the ‘Cycle Threshold’(Ct) value. Understanding Ct values and their interpretation in the context of laboratory testing is of particular importance to public health practitioners. It is a numerical value generated during an RT-PCR test, cycle number at which the fluorescence generated within a reaction crosses the fluorescence threshold, a fluorescent signal significantly above the background fluorescence. At the threshold cycle, a detectable amount of amplicon product has been generated during the early exponential phase of the reaction. Also, it refers to the number of cycles needed for a sample to amplify and cross a threshold (cut-off) to be considered detected/positive. Most RT-PCR tests use Ct cutoffs of 35-40 cycles, so any sample with a Ct value below the cutoff would be considered a true positive.
Ct (threshold cycle) is the intersection between an amplification curve and a threshold line. It is a relative measure of the concentration of the target in the PCR reaction. Also, CT value is a type of virus concentration. The higher the concentration, the lesser the number of cycles needed for the virus to be detected. It is believed that if the sample concentration is higher, then the CT value will be less, indicating that the sample is more contagious.
During clinical interpretation, understanding, and investigation, some doctors believe that a lower Ct value indicates high viral load in the patient's body. Besides, Ct values enable doctors to know the severity of symptoms the patient may develop as well as help them make their decision on whether the patient can remain isolated at home or should be admitted to a hospital.
On the other hand, some experts and professors believe that there is no tangible proof of the correlation between Ct values and the severity of the disease. It is being claimed in many news reports that even if the report is positive for the RT-PCR test, patients who have a higher CT value will not spread the infection. In contrast, there are also cases with a higher CT value that are more likely to spread the virus than an infected person with a lower CT value who stays isolated. Samples from asymptomatic/mild cases show Ct values similar to those who develop severe disease. In such a situation, fixing the cutoff can mislead people. Unfortunately, there are no accurate guidelines formulated by the Government of Nepal concerning the Ct value. It is believed that estimating infectivity based on the CT value may prove to be wrong and may lead to a false perception of safety. It is not recommended for COVID-19 patients to rely on numerical CT values to determine infectiousness and patient management protocols.
Many factors impact the absolute value of Ct besides the concentration of the target. The fluorescence emission is also dependent on environmental factors such as the pH of a solution, and salt concentration that is present in the master mixture. However, Ct values differ from one kit to another. Comparability of Ct values among different kits is a challenge as our labs are using a mixed basket of kits now with different Ct cut-offs and different gene targets. Ct values are also depending on how the sample has been collected. A poorly collected sample may reflect inappropriate Ct values.
Besides, Ct values are also determined by the technical competence of the person performing the test, calibration of equipment, and pipettes and analytical skills of the interpreters. Also, Ct values between nasal and oropharyngeal specimens collected from the same individual may differ. Similarly, the temperature of transportation as well as the time taken from collection to receipt in the laboratory can also adversely impact Ct values. The seriousness of COVID-19 depends on host factors besides the viral load.
Some patients with low viral load may land up in very severe diseases due to the activation of the immunological reactions. Hence, again high Ct value may give a false sense of security. Moreover, the RT-PCR test presently being conducted is qualitative. Ct values may give a rough estimate of the viral load. However, more specialized standards are required for quantitative assays which are currently unavailable for SARS-CoV-2.
Nevertheless, Nepal has to enhance testing and strengthen tracing, isolation, and quarantine mechanisms and care of COVID-19 patients as it is in a risk zone because of the comparably weak health system.
(The author is a research officer at Kathmandu Center for Genomics and Research Laboratory.)
You May Like This

China issues white paper on country's battle against COVID-19
BEIJING, June 7: China has issued a white paper entitled "Fighting COVID-19: China in Action" on the country's battle against... Read More...
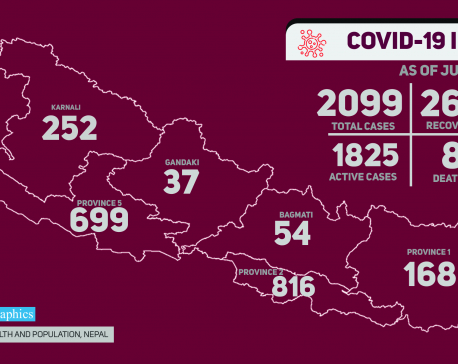
Number of COVID-19 cases in Nepal crosses 2000 mark
KATHMANDU, June 2: The number of COVID-19 cases in Nepal crossed 2000 mark on Tuesday with the highest addition on... Read More...

Leaders demand mandatory quarantine for people from coronavirus-hit countries
KATHMANDU, March 12: Major political parties have urged the government to adopt extra precautions against the risk of COVID-19 outbreak... Read More...






Just In
- Russia warns NATO nuclear facilities in Poland could become military target
- 16th Five Year Plan: Govt unveils 40 goals for prosperity (with full list)
- SC hearing on fake Bhutanese refugees case involving ex-deputy PM Rayamajhi today
- Clash erupts between police and agitating locals in Dhanusha, nine tear gas shells fired
- Abducted Mishra rescued after eight hours, six arrested
- Forest fire destroys 13 houses in Khotang
- First meeting of Nepal-China aid projects concludes
- Lungeli appointed as Minister for Labor and Transport in Madhesh province govt












Leave A Comment