
OR
Phase III clinical trials of three COVID-19 vaccines to start soon in Nepal
Published On: August 24, 2020 09:15 AM NPT By: Republica | @RepublicaNepal
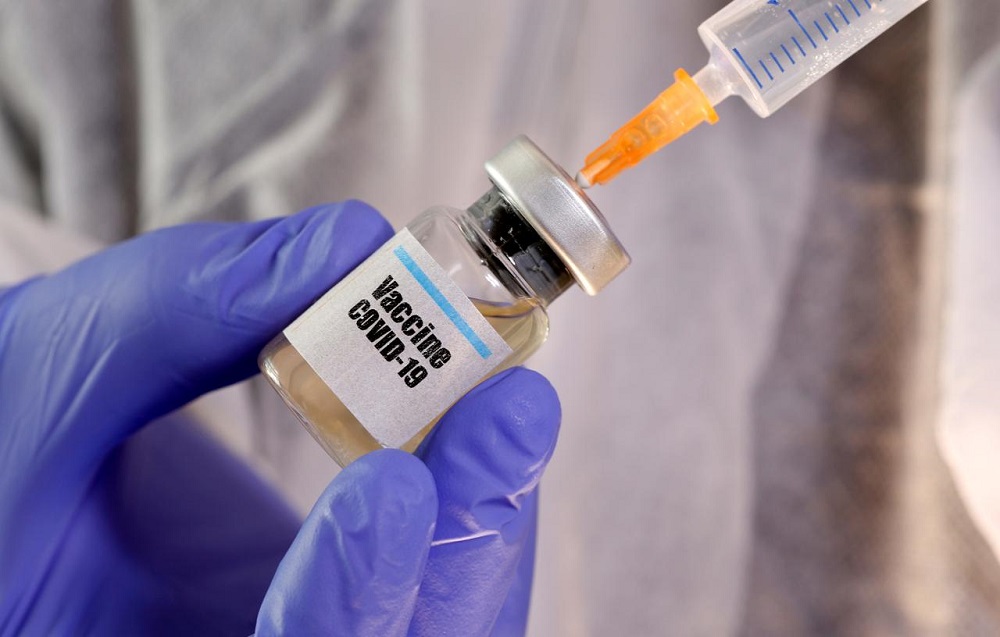
Three possible vaccines against COVID-19 positive to conduct clinical trials in Nepal
KATHMANDU, Aug 24: The Nepal Health Research Center has started preparing for clinical trials of investigational vaccines being developed against COVID-19 in Nepal.
After getting a green signal from the Ministry of Health and Population (MoHP) on Sunday, the NHRC has started preparations for phase III clinical trials for vaccines – developed by researchers in different parts of the world –against COVID-19 in Nepal.
"The NHRC has started preparations for clinical trials for vaccines against COVID-19 following a green signal from the health ministry. We [NHRC] are preparing for phase III clinical trials of three vaccines developed in the United Kingdom, China, and Russia," Pradip Gyawali, member secretary at the NHRC, told Republica.
"The NHRC is discussing clinical trials of Covidshield—a vaccine developed by Oxford University— in Nepal. We [NHRC] have discussed with the World Health Organization (WHO) about phase III trials of Covishield in Nepal. The Oxford university will finalize the clinical trial of Covishield in Nepal within a week," said Gyawali.
Likewise, the Russian Embassy in Nepal is also positive for a phase III clinical trial of a possible vaccine developed against COVID-19 in Nepal. The Russian Embassy is holding several meetings with the concerned government authorities for the clinical trial of "Sputnik-V" – a possible vaccine against COVID-19 developed in Russia.
Although the government of Russia has already approved "Sputnik-V", the vaccine is yet to go through a phase III clinical trial. "Though the vaccine is already approved, no phase III has been conducted. Russia is positive about conducting clinical trials in Nepal. But, the government will study about the vaccine before clinical trials," Gyawali said.
Likewise, the Honghi Group is also positive about conducting trials of a vaccine developed in China in Nepal. The group wants to conduct trials in at least 1,000 volunteers, according to NHRC.
However, the NHRC is yet to set a protocol for conducting clinical trials. "The government is preparing to set a protocol as several companies will show interest for clinical trials," said Gyawali.
According to the World Health Organization, it has tracked more than 170 possible vaccines being developed in different parts of the world. Of them, 138 are in pre-clinical stage – not tested in humans yet. Likewise, 25 are in phase I, 15 in phase II, and 7 in phase III of clinical trials.
You May Like This
Covid-19 vaccines given by China as grant expire unused
KATHMANDU, Jan 3: A total of 40,000 vaccines provided by the Chinese government as a grant to the Government of... Read More...

Vaccine deliveries rising as delta virus variant slams Asia
JAKARTA, July 16: As many Asian countries battle their worst surge of COVID-19 infections, the slow flow of vaccine doses from... Read More...

Costa Rica signs COVID-19 vaccine deal with Pfizer and BioNTech
SAN JOSE, Dec 4: Costa Rica has signed an agreement with pharmaceutical companies Pfizer Inc and its German partner BioNTech... Read More...





Just In
- Challenges Confronting the New Coalition
- NRB introduces cautiously flexible measures to address ongoing slowdown in various economic sectors
- Forced Covid-19 cremations: is it too late for redemption?
- NRB to provide collateral-free loans to foreign employment seekers
- NEB to publish Grade 12 results next week
- Body handover begins; Relatives remain dissatisfied with insurance, compensation amount
- NC defers its plan to join Koshi govt
- NRB to review microfinance loan interest rate











Leave A Comment