
OR
Login using
Although China has announced to provide COVID-19 vaccine to Nepal, Sinopharm yet to submit necessary documents to govt for emergency approval
February 3, 2021 06:30 am
The government has already asked the Chinese company to submit the necessary documents including phase III trial data of the vaccine
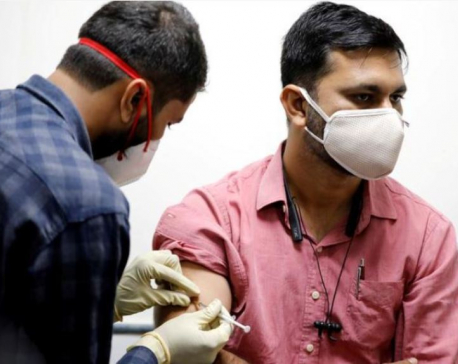
India's approval of homegrown vaccine criticised over lack of data
January 4, 2021 09:02 am
NEW DELHI, Jan 4: India on Sunday granted emergency approval to Bharat Biotech’s COVAXIN but faced questions from industry experts and opposition lawmakers after taking the step without publishing efficacy data for the homegrown coronavirus vaccine.
Trending
Just In
- Nepalgunj ICP handed over to Nepal, to come into operation from May 8
- Nepal to gift two elephants to Qatar during Emir's state visit
- NUP Chair Shrestha: Resham Chaudhary, convicted in Tikapur murder case, ineligible for party membership
- Dr Ram Kantha Makaju Shrestha: A visionary leader transforming healthcare in Nepal
- Let us present practical projects, not 'wish list': PM Dahal
- President Paudel requests Emir of Qatar to initiate release of Bipin Joshi
- Emir of Qatar and President Paudel hold discussions at Sheetal Niwas
- Devi Khadka: The champion of sexual violence victims





_20240423174443.jpg)





