
OR
Here's what scientists now want to know about COVID vaccine
Published On: December 4, 2020 08:20 PM NPT By: Agencies
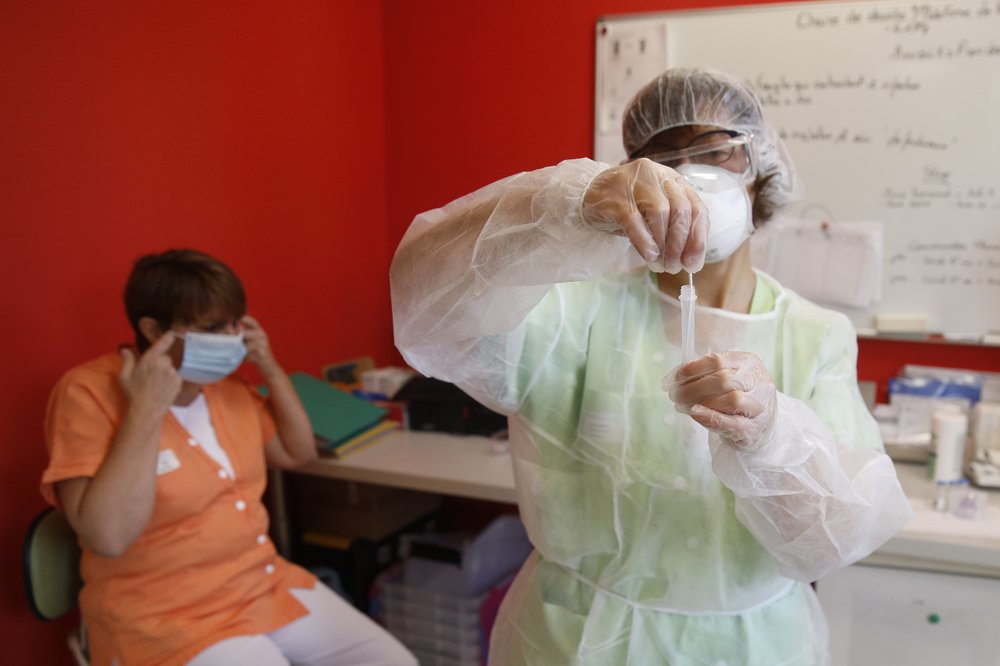
KATHMANDU, Dec 4: With striking speed, the United Kingdom has become the first country to approve a COVID-19 vaccine that has been tested in a large clinical trial. On 2 December, UK regulators granted emergency-use authorization to a vaccine from drug firms Pfizer and BioNTech, just seven months after the start of clinical trials. British front-line health-care workers, as well as care-home staff and residents, could receive their first doses next week.
China and Russia had approved vaccines already, but before they’d completed the final round of tests in people. Regulators in the United States and Europe are expected to issue their decisions in the coming weeks.
Tests on more than 43,000 people have shown that the Pfizer vaccine is 95% effective at preventing disease when measured a week after its second dose, the New York City-based firm said in November when it and BioNTech, in Mainz, Germany, submitted a request for emergency approval to the US Food and Drug Administration. The UK approval is based on data from only 170 infections, and real-world efficacy might be lower than in a trial, but it is still an extraordinarily promising result, says immunologist Danny Altmann of Imperial College London: “This is brilliant news.”
The approval is a historic moment. But scientists still have many questions about how this and other vaccines will perform as they’re rolled out to millions of people.
Do the vaccines prevent transmission of COVID-19?
In addition to the Pfizer vaccine, regulators are poring over data from another similar vaccine made by Moderna of Cambridge, Massachusetts, and a third vaccine produced by AstraZeneca of Cambridge, UK, and the University of Oxford, UK. All three of these vaccines have been tested in large clinical trials, and have shown promise in preventing disease symptoms.
But none has demonstrated that it prevents infection altogether, or reduces the spread of disease in a population. This leaves open the chance that those who are vaccinated could remain susceptible to asymptomatic infection — and could transmit that infection to others who remain vulnerable. “In the worst case scenario, you have people walking around feeling fine, but shedding virus everywhere,” says virologist Stephen Griffin of the University of Leeds, UK.
Pfizer has said that its scientists are looking at ways to assess disease transmission in future studies. For now, AstraZeneca and the University of Oxford may be able to provide the first hints as to whether a vaccine can protect against such transmission. Although they have yet to publish complete results, their trial did test participants routinely for SARS-CoV-2, allowing investigators to pick up on asymptomatic infections. Early indications are that the vaccine may have reduced the frequency of such infections. That would suggest that disease transmission might also be reduced.
How long will vaccine-induced immunity last?
There is no quick way to determine how long immunity to the SARS-CoV-2 virus will last, and researchers will need to monitor this closely in the coming months and years.
There have been some reports of reinfection and falling antibody levels months after an initial bout of COVID-19, but it is still unclear how prevalent reinfection is. And there are signs that the immune system preserves a memory of coronavirus infection in the form of specialized memory cells that could kick into action rapidly if the virus is encountered again. And vaccines, Altmann says, are deliberately designed to provoke strong responses from the immune system.
Still, it will be important for public health officials to monitor immunity – and to know when it begins to wane. One way to do that, in addition to keeping track of infections among people who have received the shots, is to periodically assess levels of antibodies and immune cells.Tracking the kinetics of these immune responses could give an early indication of when they are waning to worrisome levels, says Altmann. But the wide variation in people’s immune responses could make it a challenge to work out the circumstances in which a vaccine doesn’t work, and such studies will need to track many people. “You need to have a good stab at some high-level population analysis to work out whether you’re winning or losing,” says Altmann. “Otherwise, you might be a government kidding yourself in years’ time.”
How well do the vaccines work in groups such as old people and children?
The major vaccine trials to date have enrolled tens of thousands of people, but their conclusions about effectiveness are drawn from infections in fewer than 200 of them. As a result, it can be difficult to break up that data into different groups — such as people who are obese or elderly — without losing statistical power. “We need to see more data in terms of effects of vaccines across different demographics,” says Michael Head, an infectious disease researcher at the University of Southampton, UK.
There are early indications that the three leading vaccines may protect people over 65. But it will likely require real-world data from large numbers of vaccinated people before researchers can achieve the demographic granularity needed to ensure that parts of the population aren’t left unprotected.
There is no data yet on how the vaccine fares in children and pregnant women. Such trials often lag behind tests in other groups of people, to ensure that as much safety data as possible has been collected before those studies begin. On 2 December, Moderna unveiled plans to test its vaccine in children.
How do the vaccines stack up against each other?
All three leading vaccines have probably beaten the goal of achieving 50% efficacy, and all look to be safe, based on the clinical trial data so far. But there might be differences in how well they work, and in which contexts, that could shape the course of the pandemic.
Vaccines from Pfizer and Moderna rely on RNA, encased in a lipid particle that ferries the RNA into cells where it is used as a template to generate a viral protein that stimulates the immune system. AstraZeneca’s vaccine, however, uses DNA that is shuttled into cells within a harmless virus, unrelated to coronavirus.
Early data suggest that the RNA approach may be more effective for preventing disease. But there are subtle differences in the immune response provoked by each approach, notes Griffin. Researchers might eventually find that one approach works better than another in certain groups of people, or that one is the best at limiting transmission.
The difference in cost and logistics will also shape which vaccine is best for which region. Shortly after announcing the authorization of the Pfizer vaccine, UK officials acknowledged that getting the vaccine to individual care homes to vaccinate residents would be a challenge because the vaccine needs to be stored at extremely low temperatures (–70C). The other two vaccines do not need to be kept at such low temperatures, and the AstraZeneca vaccine is likely to be the easiest and cheapest to store of the three, says Head.
Comparisons between the effectiveness of the different vaccines are important and should be done, but until then, the path forward is clear, says Altmann. “Grab any vaccine that your government can buy,” he says. “All I want is out of this mess and my family not to be in danger, and any one of the vaccines that we’re talking about will get us there.”
Could the virus evolve to evade immunity given by vaccines?
Some viruses, like the wily influenza virus, are notorious for mutating and moving around bits of their genomes. The SARS-CoV-2 genome, however, so far appears to be fairly stable. Most of the vaccines being developed, including the three that lead the pack, target a protein called spike that the virus needs to infect cells. And immune responses elicited by those vaccines will likely target multiple sites along that protein.
All of this gives researchers some reassurance that the virus might not evolve ways to evade immunity conferred by vaccines. But mass vaccination campaigns will, for the first time, put enormous pressure on SARS-CoV-2 to adapt, and will select for any strain of the virus that might be able to escape immune defences. “We’ve never seen a virus like this under selective pressure,” says Griffin. “So we don’t know how it’s going to respond.”
As a result, researchers will need to monitor SARS-CoV-2 isolates for signs of change, says Charlie Weller, head of vaccines at the Wellcome Trust in London. “Robust surveillance with ongoing sampling and sequencing will be key to assessing any potential impact on public health and picking up any mutations,” she says, “as will the continued research in developing the next generation of Covid-19 vaccines.”
It will be handy to have vaccines against other targets ready, in case they need to be deployed against a SARS-CoV-2 that has become resistant to the vaccines that target sSpike, says Altmann. “It’s not high up on my list of panics,” he says. “But never say never: there could be emergent versions where we’d need to have vaccines against other targets up our sleeves.”
How will scientists monitor for long-term safety concerns?
“The public’s safety has always been at the forefront of our minds,” says a statement from June Raine, chief executive of the MHRA, that accompanied the UK approval of the Pfizer vaccine.
The vaccine has only completed a few months of its two-year clinical trial period — which it will need to complete before it is approved to be sold freely on the market — so health officials, clinicians and people receiving the vaccine will be watching closely for as-yet unobserved signs of danger. Many governments already have reporting systems in place that are designed to track vaccine safety.
Vaccines are rigorously vetted for potential side effects in clinical trials that combine self-reporting from participants and data collection by research clinicians. The Pfizer–BioNTech vaccine is given in two doses at least three weeks apart. For a week after each dose, participants keep tabs on their health status using an electronic diary or smartphone app. Blood is taken the day after a dose is given and one week after each injection to look for anything that might indicate a dangerous reaction.
Pfizer’s trials revealed that some recipients experienced pain at the injection site, fever, fatigue, sore muscles and headaches — although these symptoms usually lasted only a few days and are generally not considered serious. But they can stoke fear. “When the reaction to the vaccine and to the disease have the same characteristics, people get worried,” says Jerome Kim, director-general of the International Vaccine Institute in Seoul.
MHRA’s assurance that the vaccine is safe is based on monitoring hundreds of patients for at least two months after their second dose. By then, serious complications usually don’t happen, says Kim.
But after a vaccine is approved, whether with full approval or only for emergency use, clinicians are expected to continue reporting any adverse reactions. Most countries have some kind of agency, such as the US Vaccine Adverse Event Reporting System (VAERS), which collects reports of serious symptoms that occur after people receive a vaccine. US doctors are legally bound to report such symptoms. For COVID-19 drugs and vaccines, the United Kingdom has set up a specialized Coronavirus Yellow Card Reporting Site for such data collection.
Kim says such systems work. “You still need strong surveillance. These rare events can be important,” he says.
(This piece written by Heidi Ledford, David Cyranoski & Richard Van Noorden is adapted from nature.com)
You May Like This
Medics who received second dose of Covishield vaccine test positive for COVID-19
KATHMANDU, April 20: While the Nepal government is administering the second dose of Covishield vaccine to those who had received... Read More...
Moderna to charge $25-$37 for COVID-19 vaccine: CEO tells paper
FRANKFURT, Nov 22: Moderna will charge governments between $25 and $37 per dose of its COVID-19 vaccine candidate, depending on... Read More...

Oxford COVID-19 vaccine trials produce robust immune response in elderly
LONDON, Oct 26: Early results from tests for a coronavirus vaccine being developed by the University of Oxford, in collaboration... Read More...




Just In
- Govt receives 1,658 proposals for startup loans; Minimum of 50 points required for eligibility
- Unified Socialist leader Sodari appointed Sudurpaschim CM
- One Nepali dies in UAE flood
- Madhesh Province CM Yadav expands cabinet
- 12-hour OPD service at Damauli Hospital from Thursday
- Lawmaker Dr Sharma provides Rs 2 million to children's hospital
- BFIs' lending to private sector increases by only 4.3 percent to Rs 5.087 trillion in first eight months of current FY
- NEPSE nosedives 19.56 points; daily turnover falls to Rs 2.09 billion















Leave A Comment