
OR
Unrecoverable ozone layer loss will expose billions to cancer causing UV rays
Published On: February 6, 2018 04:20 PM NPT By: Agencies

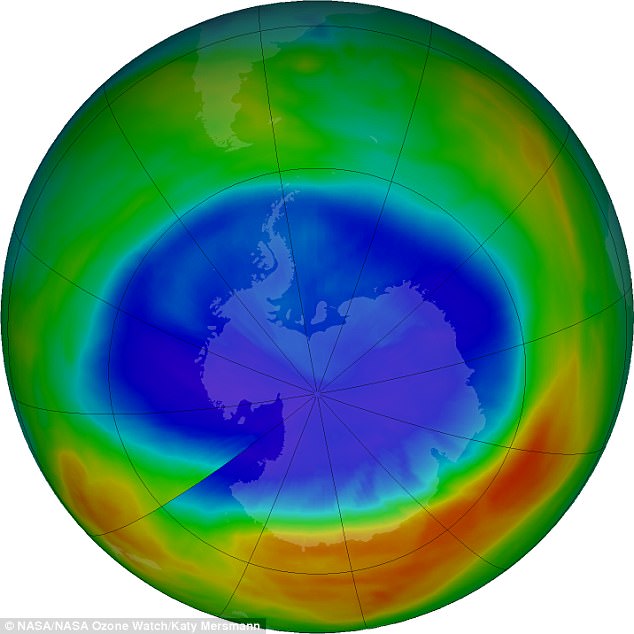
Hopes the ozone layer is recovering have been dashed by new research showing it continues to decline in the most populated areas of the world.
Ozone levels have shown signs of recovery around the poles since efforts in the 1980s to curb the use of damaging chemicals.
But scientists have now discovered the ozone layer - a substance which absorbs UV radiation from the sun - is failing to recover at lower latitudes where the majority of people live.
This could place billions of people at risk of being exposed to increasingly powerful cancer-causing UV rays, especially towards the equator, where sunlight is stronger.
Study co-author Professor Joanna Haigh, Co-Director of the Grantham Institute for Climate Change and the Environment at Imperial College London, said: 'Ozone has been seriously declining globally since the 1980s.
 'But while the banning of CFCs is leading to a recovery at the poles, the same does not appear to be true for the lower latitudes.
'But while the banning of CFCs is leading to a recovery at the poles, the same does not appear to be true for the lower latitudes.
'The potential for harm in lower latitudes may actually be worse than at the poles', she said.
The ozone forms at tropical latitudes of the stratosphere, which is the region of the atmosphere between six and 31 miles (10km and 50km) altitude, before spreading around the globe.
'The decreases in ozone are less than we saw at the poles before the Montreal Protocol was enacted, but UV radiation is more intense in these regions and more people live there', said Dr Haigh.
The Montreal Protocol was agreed in 1987 to phase out damaging CFC chemicals, used in everything from aerosols to refrigerators.
Studies have shown clear signs of recovery around the Antarctic, where an ozone 'hole' had formed, and elsewhere.
But the new study, published in Atmospheric Chemistry and Physics, found it is likely not recovering at latitudes between 60°N and 60°S (London is at 51°N).
WHAT IS OZONE LAYER?
Ozone is a molecule comprised of three oxygen atoms that occurs naturally in small amounts.
In the stratosphere, roughly seven to 25 miles above Earth's surface, the ozone layer acts like sunscreen, shielding the planet from potentially harmful ultraviolet radiation that can cause skin cancer and cataracts, suppress immune systems and also damage plants.
It is produced in tropical latitudes and distributed around the globe.
 Closer to the ground, ozone can also be created by photochemical reactions between the sun and pollution from vehicle emissions and other sources, forming harmful smog.
Closer to the ground, ozone can also be created by photochemical reactions between the sun and pollution from vehicle emissions and other sources, forming harmful smog.
Although warmer-than-average stratospheric weather conditions have reduced ozone depletion during the past two years, the current ozone hole area is still large compared to the 1980s, when the depletion of the ozone layer above Antarctica was first detected.
This is because levels of ozone-depleting substances like chlorine and bromine remain high enough to produce significant ozone loss.
In the 1970s, it was recognised that chemicals called CFCs, used for example in refrigeration and aerosols, were destroying ozone in the stratosphere.
In 1987, the Montreal Protocol was agreed, which led to the phase-out of CFCs and, recently, the first signs of recovery of the Antarctic ozone layer.
The upper stratosphere at lower latitudes is also showing clear signs of recovery, proving the Montreal Protocol is working well.
But the new study, published in Atmospheric Chemistry and Physics, found it is likely not recovering at latitudes between 60°N and 60°S (London is at 51°N).
The cause is not certain but the researchers believe it is possible climate change is altering the pattern of atmospheric circulation - causing more ozone to be carried away from the tropics.
They say another possibility is that very short-lived substances (VSLSs), which contain chlorine and bromine, could be destroying ozone in the lower stratosphere.
VSLSs include chemicals used as solvents, paint strippers, and as degreasing agents.
One is even used in the production of an ozone-friendly replacement for CFCs.
The cause is not certain but the researchers believe it is possible climate change is altering the pattern of atmospheric circulation - causing more ozone to be carried away from the tropics.
They say another possibility is that very short-lived substances (VSLSs), which contain chlorine and bromine, could be destroying ozone in the lower stratosphere.
VSLSs include chemicals used as solvents, paint strippers, and as degreasing agents.
One is even used in the production of an ozone-friendly replacement for CFCs.
Dr William Ball, who led the analysis, said: 'The finding of declining low-latitude ozone is surprising, since our current best atmospheric circulation models do not predict this effect.
'Very short-lived substances could be the missing factor in these models.'
To conduct the analysis the team developed new algorithms to combine the efforts of multiple international teams who have worked to connect data from different satellite missions since 1985.
They used the data to 'create a robust, long time series'.
Dr Ball added: 'The study is an example of the concerted international effort to monitor and understand what is happening with the ozone layer; many people and organisations prepared the underlying data, without which the analysis would not have been possible.'
Although individual datasets had previously hinted at a decline, the application of advanced merging techniques and time series analysis revealed a longer-term trend of ozone decrease in the stratosphere at lower altitudes and latitudes.
Researchers say the focus now should be on getting more precise data on the ozone decline.
They also say it is important to determine what the cause most likely is, for example by looking for the presence of VSLSs in the stratosphere.
Dr Justin Alsing, who worked on the statistical technique of the study, said: 'This research was only possible because of a great deal of cross-disciplinary collaboration.
'My field is normally cosmology, but the technique we developed can be used in any science looking at complex datasets.'
You May Like This

Upper Tamakoshi delay causing nation daily loss of Rs 40 million
DOLAKHA, April 12: The country is suffering a loss of around Rs 1.2 billion per month due to delay in the... Read More...

Loss of heritage is loss of living culture
KATHMANDU, Nov 28: The idols of goddess Bal Kumari and Rudryani from the Rudrayani Temple in Khokana was stolen on the... Read More...

Iniesta returns as Spain seeks to avenge loss to Italy
MADRID, Sept 30: Andres Iniesta will lead Spain in its World Cup qualifying matches against Italy and Albania after returning from... Read More...



Just In
- 352 climbers obtain permits to ascend Mount Everest this season
- 16 candidates shortlisted for CEO position at Nepal Tourism Board
- WB to take financial management lead for proposed Upper Arun Project
- Power supply to be affected in parts of Kathmandu Valley today as NEA expedites repair works
- Godepani welcomes over 31,000 foreign tourists in a year
- Private sector leads hydropower generation over government
- Weather expected to be mainly fair in most parts of the country today
- 120 snow leopards found in Dolpa, survey result reveals






_20220508065243.jpg)








Leave A Comment