
OR
Govt tells drug manufacturers and dealers not to sell medicines containing Ethylene Glycol and Di-ethylene Glycol
Published On: November 29, 2022 07:43 PM NPT By: Republica | @RepublicaNepal

KATHMANDU, Nov 29: The government has instructed pharmaceutical companies and drug dealers not to manufacture and sell the medicines that contain Ethylene Glycol and Di-ethylene Glycol compounds.
Expressing concerns over the health hazards caused by imported medicines having these compounds, the Department of Drug Administration (DDA) has issued a public notice in this regard. According to the DDA, a number of medicinal products, which are imported from India, have been found containing these harmful compounds. These medicines are mostly used for baby cures.
The World Health Organization (WHO) has also banned using these chemicals. According to the international organization, these chemicals can permanently damage the human kidneys causing the death of the users.
Promethazine Oral Solution, Coughex Mylene Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup manufactured by Maiden Pharmaceuticals Limited, Haryana, India, have been tested with the harmful substances. Since last month, the Indian government has also halted all production of the pharmaceutical company on charge of manufacturing fatal drugs.
According to the DDA, many Nepali drug manufacturers have also been importing raw materials like Propylene Glycol, Polyethylene Glycol, Sorbitol and Glycerin and Glycerol, among others, to prepare medicines. The regulator has asked the companies to manufacture their products only after making sure that these raw materials are free from contamination of Ethylene Glycol and Di-ethylene Glycol.
You May Like This
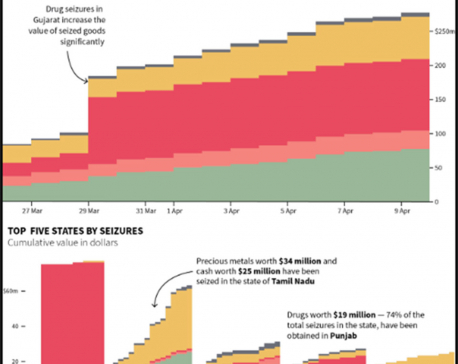
India gold smuggling slowed by election seizures of cash, bullion
MUMBAI, April 15: India’s gold smugglers have slowed their operations over worries their shipments will be caught up in seizures... Read More...

Youths opt for controlled drugs over hard drugs
KATHMANDU, Dec 15: Bijay Tamang of Kamalamai municipality in Sindhuli district put on a reluctant smile and pointed to his right... Read More...

Drugs seizure prompts DDA to tighten locker rules
KATHMANDU, Sept 30: Following the misuse of Department of Drug Administration (DDA) locker facility by a representative of pharmaceutical company, the... Read More...



Just In
- 352 climbers obtain permits to ascend Mount Everest this season
- 16 candidates shortlisted for CEO position at Nepal Tourism Board
- WB to take financial management lead for proposed Upper Arun Project
- Power supply to be affected in parts of Kathmandu Valley today as NEA expedites repair works
- Godepani welcomes over 31,000 foreign tourists in a year
- Private sector leads hydropower generation over government
- Weather expected to be mainly fair in most parts of the country today
- 120 snow leopards found in Dolpa, survey result reveals






_20220508065243.jpg)








Leave A Comment