
OR
#COVID-19 Pandemic
Govt issues licenses to import 55,000 vials of Remdesivir to treat COVID-19 patients
Published On: May 1, 2021 06:10 PM NPT By: Republica | @RepublicaNepal
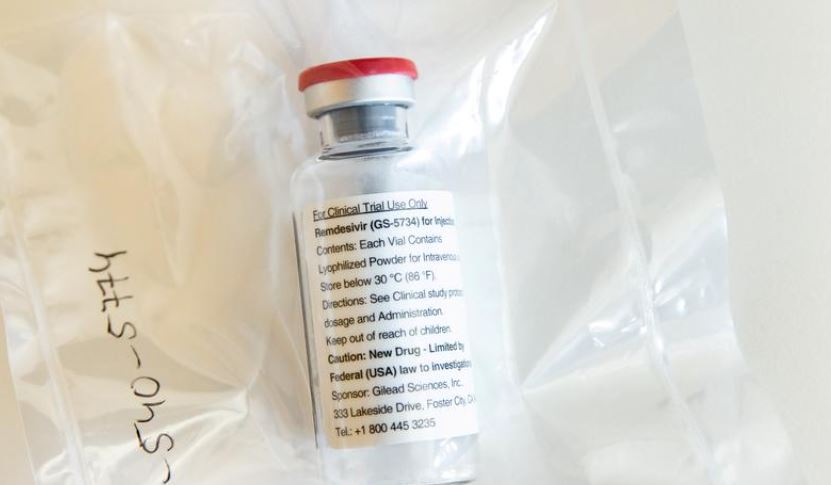
KATHMANDU, May 1: The government has permitted four companies to import 55,000 vials of Remdesivir for the treatment of COVID-19 patients.
Remdesivir is an investigational broad-spectrum antiviral agent, which has been proven efficient in vitro activity against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). It is now considered as a potential therapeutic agent against COVID-19 that is helpful mainly to the patients who need ventilator support to fight against the pandemic.
With soaring cases of the coronavirus infections in the past few weeks, the government has sought to increase the supply of the medicine that is considered to save the lives of COVID-19 patients. According to the Department of Drug Administration, the aforementioned quantity of the medicine is expected to enter Nepal in the next one week. Last week, the regulator issued licenses to import 30,000 vials of Remdesivir from Bangladesh.
In contrast to the government report, the World Health Organization (WHO), however, has not strongly recommended using the medicine to treat COVID-19 patients. Last November, the WHO through a caution notice, issued a conditional recommendation against the use of Remdesivir in hospitalized patients, stating there was no evidence that Remdesivir improves survival and other outcomes in these patients.
But a study conducted by Nepal Health Research Council (NHRC) in February this year shows that the medicine has been effective in treating COVID-19 patients. The NHRC in its study report stated that 84 percent of the patients – moderate and serious ones – recovered and were discharged after getting the antiviral drug.
You May Like This

Although China has announced to provide COVID-19 vaccine to Nepal, Sinopharm yet to submit necessary documents to govt for emergency approval
The government has already asked the Chinese company to submit the necessary documents including phase III trial data of the... Read More...

As Oli government limits its role to counting deaths during the greatest public health crisis, people are dying at an alarming rate
Experts say the government has decided to shred the constitution ... Read More...

Nepalgunj Medical College Teaching Hospital sealed off
NEPALGUNJ: The Kohalpur-based Nepalgunj Medical College Teaching Hospital has been sealed off after a patient tested positive for COVID-19 on... Read More...




Just In
- Health ministry to conduct ‘search and vaccinate’ campaign on May 13
- Indian customs releases trucks carrying Nepali tea, halted across Kakarbhitta
- Silent period for by-election to begin from midnight
- SC issues short-term interim order to govt and TU not to take immediate action against TU legal advisor Khanal
- National consultation workshop advocates to scale up nutrition smart community in Nepal
- Patan High Court issues short-term interim order to halt selection process of NTB’s CEO
- NEPSE inches up 0.15 points; daily turnover increases to Rs 2.53 billion
- Bagmati Govt mandates tri-lingual signboards in offices













Leave A Comment