
OR
Forget remdesivir, GS-441524 presents a unique opportunity for Nepal
Published On: August 18, 2020 10:49 AM NPT By: Sunada Khadka

Sunada Khadka
Khadka is a Ph.D. candidate at MD Anderson Cancer Center, The University of Texas,USAnews@myrepublica.com
More from Author
SARS-CoV-2 has emerged as a worldwide public health crisis and embattled not only the countries that are at the frontiers of medicine and research, but also developing countries like Nepal. With no effective vaccine against the virus developed to date, experts expect the toll of the COVID-19 pandemic to persist. The grim forecast is deeply concerning for Nepal, where the COVID-19 cases continue to surge, and the state remains unequipped to treat the afflicted patients and reduce community transmission.
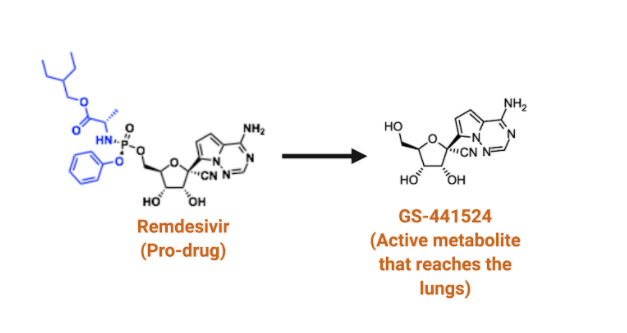
Remdesivir, a drug licensed under the American pharmaceutical company Gilead Sciences, was initially developed to treat hepatitis in 2009. In May 2020, based on the evidence that remdesivir (GS-5734) accelerated the recovery rates of SARS-CoV-2 patients, the US Food and Drug Administration granted emergency use authorization against COVID-19. While remdesivir has emerged as just one of two drugs that has shown efficacy against COVID-19, its structural complexity makes it difficult to mass produce and distribute. These shortcomings significantly dampen its clinical utility. Dr. Florian Muller and Victoria Yan, two medicinal chemistry experts, affiliated with MD Anderson Cancer Center in the US, argue that its synthetic difficulty is partly attributed to remdesivir’s identity as an antiviral phosphate prodrug. This imposes significant challenges in its scalability. Pertinently, health officials in Nepal have decried the Nepal government’s effort to import remdesivir, and this has forced desperate patients to seek treatment options through black markets.
But the question remains: Is the chemical complexity of remdesivir justified? According to Public Citizen, a consumer advocacy group based in Washington DC, rallied by many American scientific experts, the chemical complexity of remdesivir is unjustified. These scientists make a case for a simpler intermediate in the remdesivir biosynthesis, GS-441524, as a better alternative drug against COVID-19. Indeed, a growing body of preclinical research highlights the efficacy of GS-441524 against SARS-CoV-2, and based on the data, it is arguable that it may have substantial advantages over remdesivir. For instance, multiple studies have suggested that it is safer and could be given at higher doses than remdesivir.
Inside the body, GS-441524 gets converted to the same active antiviral molecule as remdesivir, that blocks the replication of SARS-CoV2. Interestingly, GS-441524 has already shown dramatic clinical efficacy in veterinary setting against the lethal cat coronavirus. One significant advantage that GS-441524 has over remdesivir is that, unlike remdesivir, it is not primarily absorbed by the liver. This property, coupled with the improved water solubility of GS-441524, make oral administration possible. Oral administration of GS-441524 would enable treatment of patients with COVID-19 outside of a hospital setting, unlike remdesivir, which has to be administered intravenously at the hospital.
Despite these compelling preclinical and clinical evidence in the veterinary setting, GS-441524 is not being trialed in the US, where the virus has claimed more than 165K lives and shows no sign of abating. Public Citizen claims that Gilead has deliberately neglected the effort to trial GS-441524 against COVID-19. Gilead holds patent rights to both GS-441524 and remdesivir, but the patents rights on remdesivir will last five years longer than that for GS-441524, which allows the company to amass significant profit through remdesivir.
In the US, the regulatory framework and legislation on intellectual property (IP) protection prevent researchers from carrying out independent clinical trials with GS-441524 without the consent of Gilead. However, the situation is quite different in Nepal because the IP regulatory constraints on remdesivir or GS-441524 do not apply. This is indeed a rare example of where the burden of legislation and IP rights restricts cutting edge research in the US, which countries like Nepal could use to their advantage. Given the urgency of the situation at hand and the thousands of lives GS-441524 could save, it does not seem impossible for Nepal to capitalize on this opportunity and investigate the use of GS-441524 as a COVID-19 treatment or cure.
Khadka is a Ph.D. candidate at MD Anderson Cancer Center, The University of Texas,USA
You May Like This

Remdesivir costing Rs 5,600 being sold for more than Rs 40,000
KATHMANDU, Nov 8: Remdesivir, an antiviral medication, which has been permitted to be used as an emergency medication against COVID-19... Read More...

As Oli government limits its role to counting deaths during the greatest public health crisis, people are dying at an alarming rate
Experts say the government has decided to shred the constitution ... Read More...

Govt permits import, sales and distribution of Remdesivir
KATHMANDU, Oct 17: The government has issued permits to import, sales and distribution of Remdesivir, an anti-viral drug used in... Read More...







Just In
- Nepali Journalists win second prize at Fetisov Journalism Awards for exposing exploitation of workers in Qatar World Cup
- Devotees gather at Balaju Park for traditional ritual shower at Baisdhara (Photo Feature)
- PPMO blacklists 33 construction companies
- UK Parliament approves Rwanda deportation bill, ending weeks of legislative stalemate
- SC refuses to issue interim order in petition against Sudurpaschim province govt
- Kathmandu to host UNDP Asia Pacific regional meeting
- DoHM cautions of heat wave in West Terai and Madhesh regions for next five days
- Is former President Bhandari returning to active politics or poised for a graceful exit?








Leave A Comment